>
>
>
Innovative Solutions for Accurate and Efficient Culture Detection of Trichomonas vaginalis and Neisseria gonorrhoeae Infections
Innovative Solutions for Accurate and Efficient Culture Detection of Trichomonas vaginalis and Neisseria gonorrhoeae Infections
- By Brandon Font, Technical Services Manager and Research Scientist, Biomed Diagnostics, a DCN Dx brand
- April 23, 2024
Introduction
Sexually transmitted infections (STIs) are a significant global health burden, with an estimated 1 million new cases occurring daily worldwide (WHO, 2022). Untreated STIs can lead to severe complications such as pelvic inflammatory disease, infertility, and increased risk of HIV acquisition (Rowley et al., 2019). The increase of STI cases in recent years has highlighted the urgent need for improved diagnostic tools that accurately and efficiently detect these infections in a wide variety of clinical settings, enabling timely treatment and prevention of further transmission.
Among the most prevalent STIs are trichomoniasis, caused by the protozoan Trichomonas vaginalis, and gonorrhea, caused by the gram-negative gonococcus bacterium Neisseria gonorrhoeae, with global annual incidence rates of 156 million and 87 million cases, respectively (WHO, 2018). T. vaginalis, a parasitic protozoan, is associated with adverse pregnancy outcomes, increased risk of HIV acquisition, and pelvic inflammatory disease (Kissinger, 2015). N. gonorrhoeae, a bacterial pathogen, can lead to serious complications such as disseminated gonococcal infections prone to antibiotic treatment failure, making prompt detection and accurate treatment crucial (Wi et al., 2017).
In this article, we aim to highlight the significant contributions of Biomed Diagnostics’ InPouch TV and InTray GC devices in revolutionizing the detection and management of T. vaginalis and N. gonorrhoeae infections. By examining the unique features, performance characteristics, and potential impact of these devices, we seek to highlight their role in strengthening the diagnostic tool kit against STIs and informing public health strategies to curb the rising tide of these infections.
Innovative Diagnostic Solutions: InPouch TV and InTray GC
InPouch TV and InTray GC, developed by Biomed Diagnostics, are innovative diagnostic devices that address the limitations of traditional methods for detecting T. vaginalis and N. gonorrhoeae, respectively. These devices combine efficient sample collection, sample transport, and optimal microenvironment culturing within a single sealed system, offering significant advantages in terms of performance, ease of use, and cost-effectiveness.
The InPouch TV is a self-contained culture system that utilizes a selective, modified Diamond’s medium to support the growth of T. vaginalis while suppressing the proliferation of other microorganisms. The device contains nutrients, antibiotics, and oxygen-resistant barrier materials that create an optimal microenvironment for T. vaginalis growth. The device is inoculated with the patient sample, incubated at 37°C, and examined microscopically daily for direct evidence of live trichomonads as soon as parasite cell growth occurs (Borchardt et al., 1997).
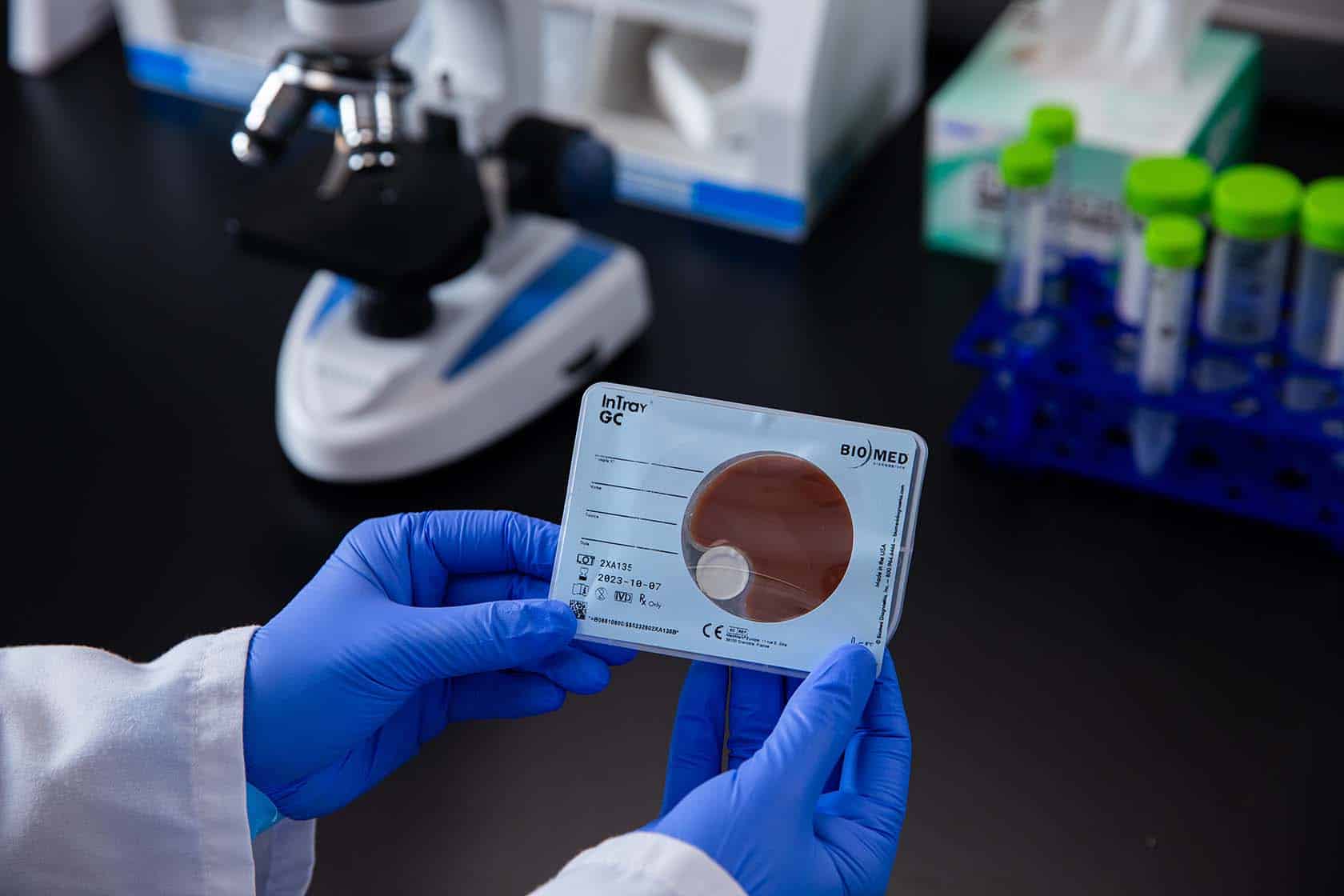
Similarly, the InTray GC employs a modified Thayer-Martin medium, which is selective for N. gonorrhoeae growth while inhibiting the growth of contaminants (Paris & Font, 2022). The InTray GC device contains antimicrobial agents, selective nutrients, and a tablet that releases CO2 gas in order to facilitate optimal growth and identification of N. gonorrhoeae colonies. After inoculation with the patient sample, the InTray GC is incubated at 35-37°C in a device generated 5% CO2 microenvironment for up to 72 hours (i.e., no need for CO2 incubator because the CO2 pellet is provided within the tray), allowing for the presumptive identification of N. gonorrhoeae based on colony morphology.
Validation studies and clinical trials have demonstrated the high accuracy and reliability of InPouch TV and InTray GC in detecting T. vaginalis and N. gonorrhoeae, respectively. In a multi-center study comparing InPouch TV with wet mount microscopy and culture, InPouch TV exhibited a sensitivity of 94.7% and a specificity of 99.4% (Borchardt et al., 1997). Similarly, a study evaluating the performance of InTray GC found a sensitivity of 100% and a specificity of 98.8% compared to standard culture methods.
Compared to traditional diagnostic methods, InPouch TV and InTray GC offer several advantages. Wet mount microscopy, the most common method for T. vaginalis detection, has a low sensitivity of 50-60%, often leading to false-negative results (Bachmann et al., 2011). InPouch TV, with its higher sensitivity and specificity, reduces the risk of missed infections and enables more accurate diagnosis. Similarly, conventional culture methods for N. gonorrhoeae require separate collection, transport, and culture steps, increasing the risk of sample contamination and delay in results (Gaydos & Hardick, 2014). InTray GC streamlines this process, providing a more efficient and reliable alternative.
Supporting Antimicrobial Resistance Surveillance and Targeted Treatment
Validation studies have also demonstrated the reliability of InPouch TV and InTray GC in transporting viable T. vaginalis and N. gonorrhoeae cells, respectively, to labs responsible for antibiotic susceptibility testing (Paris & Font, 2022). This is a crucial aspect of STI management, as the emergence of antimicrobial resistance, particularly in N. gonorrhoeae, poses a significant threat to public health (Wi et al., 2017). The ability to transport viable organisms to reference laboratories for susceptibility testing is essential for monitoring resistance patterns, informing treatment guidelines, and guiding the development of new antibiotics (Cristillo et al., 2017).
The study by Paris and Font (2022) evaluated the recovery of antimicrobial-resistant N. gonorrhoeae isolates after 72 hours of transport using the InTray GC method. The results showed that the InTray GC device successfully maintained the viability of the isolates, allowing for accurate susceptibility testing at the receiving laboratory. This finding highlights the potential of the InTray GC device to support surveillance efforts and facilitate the timely detection of antimicrobial resistance in N. gonorrhoeae (FDA 510k K210511 InTray GC).
Similarly, the InPouch TV device has been shown to maintain the viability of T. vaginalis cells during transport, enabling the successful culture and susceptibility testing of the organism at reference laboratories (Bachmann et al., 2011). This is particularly important given the increasing reports of metronidazole-resistant T. vaginalis strains, which require alternative treatment approaches (Kissinger, 2015).
The reliability of InPouch TV and InTray GC in transporting viable organisms for susceptibility testing highlights their value as comprehensive diagnostic solutions. By combining accurate detection with the ability to support antimicrobial resistance monitoring, these devices can contribute to the development of targeted treatment strategies and the containment of resistant STI strains. The integration of InPouch TV and InTray GC into STI management protocols can strengthen the overall response to the global challenge of antimicrobial resistance in STIs.
Impact on Public Health Outcomes
The widespread adoption of InPouch TV and InTray GC has the potential to significantly improve public health outcomes related to T. vaginalis and N. gonorrhoeae infections. By enabling accurate and efficient culture diagnosis, these devices can facilitate prompt treatment, reduce the duration of infectiousness, and prevent the onward transmission of these STIs (Kissinger, 2015; Wi et al., 2017).
Delayed or missed diagnoses of T. vaginalis and N. gonorrhoeae can lead to persistent infections, increasing the risk of complications and the likelihood of transmission to sexual partners (Gaydos & Hardick, 2014). The high sensitivity and specificity of InPouch TV and InTray GC can help mitigate these risks by ensuring that infected individuals are identified and treated promptly. Early detection and treatment also reduce the risk of adverse outcomes such as pelvic inflammatory disease, ectopic pregnancy, and infertility (Rowley et al., 2019).
Moreover, the cost-effectiveness of InPouch TV and InTray GC can have significant implications for healthcare systems. By reducing the need for repeat testing and follow-up visits due to false-negative results, these devices can help optimize resource utilization and reduce the overall economic burden associated with STI management (Gift et al., 2002). The simplified testing process and ease of use also make these devices suitable for use in resource-limited settings, where access to advanced diagnostic facilities may be limited (Cristillo et al., 2017).
Contribution to Global Health Targets
The adoption of InPouch TV and InTray GC can contribute to the achievement of global health targets and initiatives related to STI control and elimination. The World Health Organization (WHO) has set ambitious goals for reducing the incidence of STIs, including a 90% reduction in N. gonorrhoeae incidence by 2030 (WHO, 2016). To achieve these targets, the WHO emphasizes the need for improved diagnostic tools, increased access to testing, and strengthened surveillance systems (WHO, 2016).
InPouch TV and InTray GC align with these objectives by providing accurate, accessible, and user-friendly diagnostic solutions. The widespread use of these devices can enhance STI surveillance efforts by providing reliable data on the prevalence and distribution of T. vaginalis and N. gonorrhoeae infections. This information is crucial for guiding public health interventions, allocating resources, and monitoring progress towards STI control and elimination goals (Cristillo et al., 2017).
Furthermore, the portability and 12-month from date of manufacture stability of InPouch TV and InTray GC make them suitable for use in various healthcare settings, including primary care, outreach programs, and mobile clinics (Bachmann et al., 2011). This flexibility can help expand access to STI testing services, particularly in underserved and high-risk populations, contributing to the global efforts to reduce health disparities and ensure equitable access to STI care (Cristillo et al., 2017).
Conclusion
The global increase in STI cases, particularly T. vaginalis and N. gonorrhoeae infections, necessitates the development and widespread adoption of innovative diagnostic tools. InPouch TV and InTray GC, developed by Biomed Diagnostics, represent significant advancements in this regard. These devices combine efficient sample collection, sample transport, and optimal microenvironment culturing in a single, user-friendly system, offering high sensitivity, specificity, and rapid turnaround times compared to traditional diagnostic methods.
The evidence presented in this article highlights the potential of InPouch TV and InTray GC to support public health goals for STI reduction. Validation studies and clinical trials have demonstrated their excellent performance characteristics, with sensitivity and specificity rates exceeding 90% (Borchardt et al., 1997; Granato & Schneible, 2018). The simplified testing process and enhanced accuracy of these devices can lead to improved patient outcomes, reduced transmission rates, and cost savings for healthcare systems.
Moreover, the adoption of InPouch TV and InTray GC aligns with global health initiatives aimed at controlling and eliminating STIs. These devices can contribute to the achievement of WHO targets by enhancing STI surveillance, expanding access to testing, and guiding public health interventions (WHO, 2016). Their portability and ease of use make them suitable for various healthcare settings, including resource-limited areas, thus promoting health equity in STI care.
The development and implementation of innovative diagnostic tools, exemplified by InPouch TV and InTray GC, are crucial in the global fight against STIs. These devices have the potential to transform STI management by providing accurate, timely, and accessible diagnostic solutions in a variety of clinical settings. As the STI epidemic continues to evolve, with the emergence of antimicrobial resistance and changing epidemiological patterns, sustained investment and collaboration in the development and deployment of novel diagnostic technologies are of vital importance. By prioritizing innovation in STI diagnostics, we can strengthen our response to this global health challenge and work towards a future where STIs are effectively controlled.
References
Bachmann, L. H., Hobbs, M. M., Sena, A. C., Sobel, J. D., Schwebke, J. R., Krieger, J. N., … & Gaydos, C. A. (2011). Trichomonas vaginalis genital infections: progress and challenges. Clinical Infectious Diseases, 53(suppl_3), S160-S172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3897282/pdf/cir705.pdf
Borchardt, K. A., Smith, R. F (1991). An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Medicine, 67(2):149-52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1194652/pdf/genitmed00038-0071.pdf
Cristillo, A. D., Bristow, C. C., Peeling, R., Van Der Pol, B., de Cortina, S. H., Dimov, I. K., … & Klausner, J. D. (2017). Point-of-care sexually transmitted infection diagnostics: proceedings of the STAR sexually transmitted infection—clinical trial group programmatic meeting. Sexually Transmitted Diseases, 44(4), 211-218. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5347466/pdf/olq-44-211.pdf
FDA 510(K) Premarket Notification for InTray GC. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K210511
Gaydos, C. A., & Hardick, J. (2014). Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Review of Anti-infective Therapy, 12(6), 657-672. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4065592/pdf/nihms584921.pdf
Gift, T. L., Pate, M. S., Hook III, E. W., & Kassler, W. J. (2002). The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sexually Transmitted Diseases, 26(4), 232-240. https://journals.lww.com/stdjournal/fulltext/1999/04000/the_rapid_test_paradox__when_fewer_cases_detected.10.aspx
Kissinger, P. (2015). Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infectious Diseases, 15(1), 307. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525749/pdf/12879_2015_Article_1055.pdf
Paris KS, Font B, Mehta SR, Huerta I, Bristow CC. (2022). 72-Hour transport recovery of antimicrobial resistant Neisseria gonorrhoeae isolates using the InTray® GC method. PLoS One. 2022 Jan 21;17(1):e0259668. doi: 10.1371/journal.pone.0259668. PMID: 35061686; PMCID: PMC8782362 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8782362/pdf/pone.0259668.pdf
Rowley, J., Vander Hoorn, S., Korenromp, E., Low, N., Unemo, M., Abu-Raddad, L. J., … & Taylor, M. M. (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bulletin of the World Health Organization, 97(8), 548-562. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6653813/pdf/BLT.18.228486.pdf
WHO. (2016). Global health sector strategy on sexually transmitted infections 2016-2021: toward ending STIs. World Health Organization. https://iris.who.int/bitstream/handle/10665/246296/WHO-RHR-16.09-eng.pdf?sequence=1
WHO. (2018). Report on global sexually transmitted infection surveillance, 2018. World Health Organization. https://iris.who.int/bitstream/handle/10665/277258/9789241565691-eng.pdf?sequence=5
WHO. (2022). Sexually transmitted infections (STIs). World Health Organization. https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)
Wi, T., Lahra, M. M., Ndowa, F., Bala, M., Dillon, J. A. R., Ramon-Pardo, P., … & Unemo, M. (2017). Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Medicine, 14(7), e1002344. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5501266/pdf/pmed.1002344.pdf